|
Psychrometrics deals with thermodynamic properties
of moist air and uses these properties to analyze
conditions and process involving moist air. Atmospheric
air contains many gases components as well as
water vapor and miscellaneous contaminants (e.g.,
smoke, pollen and gaseous pollutants). Dry air
exists when all water vapor and contaminants have
been removed from atmospheric air. The composition
of dry air is relatively constant, but small variations
in the amounts of individual components occur
with time, geographic location, and altitude.
The apparent molecular mass or weighted average
molecular weight of all components, for dry air
is 28.9645, based on the carbon-12 scale. The
gas constant for dry air, based on the carbon-12
scale is 1545.32/28.9645 =53.352 ft lbf / lbm
oR.
Moist air is a binary
mixture of dry air and water vapor. The amount
of water vapor in moist air varies from zero (dry
air) to a maximum that depends on temperature
and pressure. The latter condition refers to saturation,
a state of neutral equilibrium between moist air
and the condensed water phase. Unless otherwise
stated, saturation refers to a flat interface
surface between the moist air and the condensed
phase. The molecular weight of water is 18.01528
on the carbon-12 scale. The gas constant for water
vapor is 1545.32/18.01528 = 85.778 ft lbf / lbm
oR
The temperature and
barometric pressure of atmospheric air vary considerably
with altitude as well as with local geographic
and weather conditions. The standard atmospheric
gives a standard of reference for estimating properties
at various altitudes. At sea level, standard temperature
is 59oF; standard barometric pressure
is 29.921 inch Hg. The temperature is assumed
to decrease linearly with increasing altitude
throughout the troposphere (lower atmosphere),
and to be constant in the lower reaches of the
stratosphere. The lower atmosphere is assumed
to constant of dry air that behaves as a perfect
gas. Gravity is also assumed constant at the standard
value, 32.1740 ft/s2.
Humidity ratio (alternatively,
the moisture content or mixing ratio) is defined
as the ratio of the mass of water vapor to the
mass of dry air. Specific humidity is the ratio
of the mass of water vapor to the total mass of
the moist air. Absolute humidity (alternatively,
water vapor density) is the ratio of the mass
of water vapor to the total volume of the moist
air. Saturation humidity ratio is the humidity
ratio of moist air saturated with respect to water
at the same temperature and pressure. Degree of
saturation is the ratio of the air humidity ratio
to humidity ratio of saturated air at the same
temperature and pressure. Relative humidity is
the ratio of the mole fraction of water vapor
in a given moist air to the mole fraction in an
air saturated at the same temperature and pressure.
The enthalpy of a mixture
air is the sum of the individual partial enthalpies
for dry air and for saturated water vapor at the
temperature of the mixture.
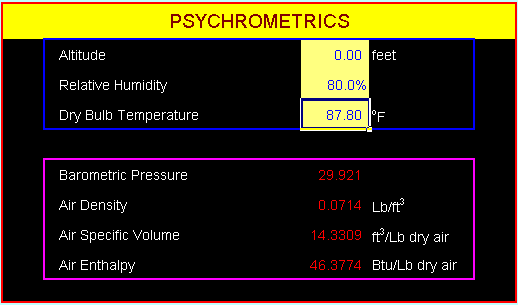
Example 1-1.
Calculate the air density, specific volume, and
enthalpy in US units at the ambient conditions
of DBT 87.8oF, RH 80% and sea level.
- Air Density: 0.0714
Lb/ft3
- Air Specific Volume:
14.3309 ft3/Lb dry air
- Air Enthalpy: 46.3774
Btu/Lb dry air
Download
the example file (exe1_1.zip)
This file covers the examples of 1-1 through 1-4.
Example 1-2.
Calculate the air density, specific volume, and
enthalpy in US at the ambient conditions of DBT
87.8oF, RH 0% (Dry Air), and sea level.
- Air Density: 0.0723
Lb/ft3
- Air Specific Volume:
13.8224 ft3/Lb dry air
- Air Enthalpy: 21.1196
Btu/Lb dry air
Example 1-3.
Calculate the air density, specific volume, and
enthalpy in US at the ambient conditions of DBT
87.8oF, RH 100%, and sea level.
- Air Density: 0.0711
Lb/ft3
- Air Specific Volume:
14.4639 ft3/Lb dry air
- Air Enthalpy: 52.9849
Btu/Lb dry air
Example 1-4.
Calculate the air density, specific volume, and
enthalpy in US at the ambient conditions of DBT
87.8oF, RH 80%, and 1,000 feet in altitude.
- Air Density: 0.0688
Lb/ft3
- Air Specific Volume:
14.8824 ft3/Lb dry air
- Air Enthalpy: 47.3494
Btu/Lb dry air
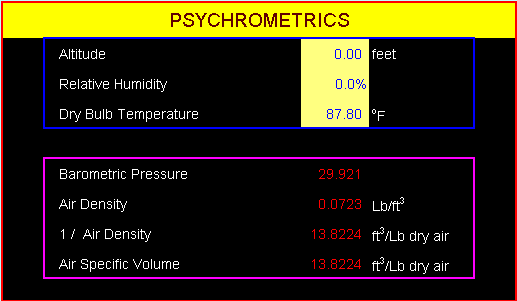
Example 1-5.
Find a relative humidity which the relationship
of 1/ air density = specific volume is established
at an ambient condition of DBT 87.8oF
and sea level.
- Air Density: 0.0723
Lb/ft3
- 1 / Air Density:
1 / 0.0723 = 13.8224 ft3/Lb dry air
- Air Specific Volume:
13.8224 ft3/Lb dry air
The relationship of
1/air density = specific volume is only valid
at the point that the relative humidity is zero.
That is, only valid for the dry air condition.
Download
the example file (exe1_5.zip) |