|
6) Breakpoint Phenomenon
The chemistry of this phenomenon
is based on the inorganic reaction of chlorine with
ammonia nitrogen. In dilute aqueous solution (1 - 50
mg/l) the reaction between ammonia nitrogen and chlorine
forms three types of chloramines in the following reactions:
HOCl + NH3 --->
NH2Cl (monochloramine) + H2O ------
Eq. 1
NH2Cl + NH3 ---> NHCl2
(dichloramine) + H2O ------ Eq. 2
NHCl2 + HOCl ---> NCl3 (trichloramiine)
+ H2O ------ Eq. 3
These reactions are in general
by steps, so that they all complete with each other.
A series of complex reactions with all of these substances
involves the chlorine substitution of each of the hydrogen
atoms in the ammonia molecule. These competing reactions
are grossly dependent upon pH, temperature, contact
time, initial chlorine to ammonia ratio, and most of
all upon the initial concentrations of chlorine and
ammonia nitrogen. Note that in all three equations the
chlorine atom is positively charged.
Above Eq. 1 will convert
all of the free chlorine to mono chloramine at pH 7
to 8 when the ratio of chlorine to ammonia is equimolar
(5:1 by weight) or less - that is, 4:1, 3:1, and so
on. The rate of this reaction is extremely important,
since it is pH-sensitive. According to reaction rates
established by Morris, the fastest conversion of HOCl
to NH2CL occurs at pH 8.3. The following
are calculated reaction rates for 99 percent conversion
of free chlorine to monochloramine at 25oC
with a molar ratio of 0.2 x 10-3 mol/l HOCl and 1.0
x 10-3 mol/l NH3:
| pH |
2 |
4 |
7 |
8.3 |
12 |
| Seconds |
421 |
147 |
0.2 |
0.009 |
33.2 |
The reaction slows appreciably
as the temperature drops. At 0oC, it requires
nearly five minutes for 90 percent conversion at pH
7. The pH dependence of this reaction is described accurately
on the basis of the HOCl- OCl-
equilibrium and the NH3- NH4 +
equilibrium.
The reaction of Eq. 2 will
form dichloramine between pH and 8 if the ratio of chlorine
to ammonia is 2 mol chlorine to one mol ammonia nitrogen
(10:1 by weight). The rate of this reaction is much
slower than that of equation 1. It may take as long
as one hour for 90 percent conversion and up to five
hours at pH 8.5 and above when ammonia nitrogen concentrations
are very low. As the pH approaches 5 the reaction speeds
up appreciably. This reaction is dependent upon pH,
initial ammonia nitrogen, and temperature. The reaction
time of Eq. 2 is known to be minute when the initial
nitrogen concentration is in excess of 1 mg/l and pH
is favorable.
The reaction of Eq. 3 will
form some trichloramine (commonly called nitrogen trichloride
when the pH is between 7 and 8 if the chlorine to ammonia
nitrogen ratio is 3 mol of chlorine to 1 mol of ammonia
nitrogen (15:1 by weight). At present very little is
known about the kinetics of this reaction, particularly
in concentrations of less than 10 ppm (10-4 M). Nitrogen
trichloride does form, even at equimolar ratios of chlorine
to ammonia nitrogen, if the pH 5, if the pH is depressed
to 5 or less. At one time it was thought that it would
not form above pH 5. It is known to exist in water treatment
plants when the pH is as high as 9. This occurs at very
high chlorine to ammonia nitrogen ratios (25:1 by weight).
In cooling water practice,
if the chemistry of Eq. 1 is practiced, it is known
as either the chlorine-ammonia process, the chloramine
process or chloramination. Eq. 2 and 3 are related to
the "breakpoint" phenomenon. It was Griffin's
work which let to the discovery of this phenomenon in
1939. Grffin was attempting to explain the sudden loss
of chlorine residuals and the simultaneous disappearance
of ammonia nitrogen at treatment plants which were experimenting
with higher than usual chlorine residuals (2 - 15 mg/l).
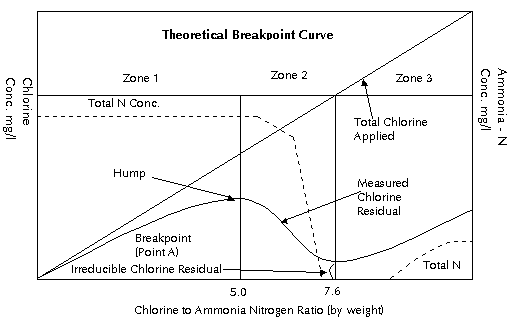
The breakpoint curve below
is a graphic representation of chemical relationships
which exist as varying amounts of chlorine are added
to waters containing small amount of ammonia nitrogen.
The theoretical breakpoint curve is shown in below.
It was originally developed as a result of Griffin's
work. This curve has several characteristic features.
The principal reaction in Zone 1 is the reaction between
chlorine and the ammonia ion indicated in Eq. 1. This
results in a chlorine residual containing only monochloramine
all the way to the hump in the curve. The hump occurs,
theoretically, at a chlorine to ammonia nitrogen weight
ratio of 5:1 (molar ratio 1:1). This ratio indicates
the point where the reacting chlorine and ammonia nitrogen
molecules are present in solution in equal numbers.
As the molar ratio begins to exceed 1:1, some of the
monochloramine starts a disproportionation reaction
to form dichloramine in accordance with Eq. 2.
To the right of the breakpoint,
Zone 3, chemical equilibria require the buildup of free
chlorine residual (HOCl). In practical applications
of breakpoint chlorination, reactions occur which result
in the formation of nitrogen gas, nitrate, nitrogen
trichloride, and other end products. These reactions
consume chlorine and cause the Cl2:NH4
+ ratio to exceed the stoichiometric value of 7.6:1
and affect the shape of the breakpoint curve.
As the chlorine to ammonia
nitrogen ratio increases beyond about 12 - 15:1 the
reaction of equation 3 sets in. Under these conditions
the formation of nitrogen trochloride will occurs even
at pH values as high as 9. As the chlorine dose is increased
beyond point A in Zone 3, the free available chlorine
residual will increase in an amount equal to the increase
in the dosage. Therefore, the breakpoint curve in Zone
3 should plot at a 45o angle.
It should be emphasized that
the shape of the breakpoint curve is affected by contact
time, temperature, concentration of chlorine and ammonia,
and pH. High concentrations increase the speed of the
reactions. As the pH decreases below 8.3, the reactions
are retarded. The higher the temperature, the faster
the reactions. The shape of the curve is different for
different contact times.
In water practice this is
known as free residual chlorination rather than the
breakpoint process. In water treatment the practical
significance of the curve is briefly as follows:
- Zone 1: The residual in
this zone up to the hump are all monochloramine. The
residuals in this zone do not form trichloramines.
- Zone 2: As the hump is
passed, the monochloramine plus the addition of more
free chlorine begins to form dichloramine, which is
about twice as germicidal as monochloramine. However,
this may not be the best part of the curve for the
production of a palatable water.
- Zone 3: At the tip of
the curve (point A) and beyond, free chlorine residual
will appear. The total residual will be made up of
the nuisance residuals plus free chlorine. If nitrogen
trichloride is formed, it will appear in this zone.
In practice it has been found that a ratio of free
chlorine to total residual of 85 percent or greater
will result in the most palatable water.
7) Alkalinity
Since chlorine solutions
are highly corrosive the application of chlorine to
a process stream will often raise the question of chlorine
corrosion. Corrosion by chlorine is related directly
to pH, which is dependent upon alkalinity. Therefore
it is appropriate to know the effect of chlorine on
the alkalinity of a water.
If a water is dosed with
chlorine to the extent of the chlorine demand of the
water, all of the chlorine applied will end up as chloride
ion (Cl-) as follows:
Cl2 + H2O
---> HOCl + HCl ------ Eq. 4
HOCl + Cl demand + HCl ---> 2HCl ------ Eq. 5
This calculate as 1.4 parts
alkalinity for each part chlorine:
2 HCl + H2O +
CaCO3 ---> CaCl2 + CO2
+ 2 H2O ------ Eq. 6
Alkalinity as CaCO3 = 100 / Cl2
= 100 / 71 = 1.4
The reaction in Eq. 6 occurs
at pH 4.3, which is the endpoint of the alkalinity titraion,
and where CO2 exists. When HOCl is not reduced,
only one Cl goes to HCl and the reaction consumes just
half of the alkalinity, or 0.7. As can be seen from
the above, the subject of alkalinity destruction by
chlorine is not a simple one.
Take the case where water
has sufficient alkalinity to maintain a 7.0 pH, and
none of the chlorine applied is consumed by chlorine
demand. In this case 50 percent of the chlorine applied
will go to HCl. Eighty percent of the remaining 50 percent
will be undissociated HOCl and unreactive in the alkalinity
reactions. However, the other 20 percent of the remaining
50 percent at pH 7 is H+ and OCl-.
The alkalinity reduction
is calculated as follows:
50% + (0.20 x 50%) = 60%
0.6 x 1.4 = 0.84 parts alkalinity
reduction by chlorine at pH 7.0. The rule of thumb for
alkalinity correction by the use of caustic to maintain
pH is a pound of caustic to a pound of chlorine. One
part of caustic produces 1.25 parts of alkalinity. If
a water at pH 7 is dosed with chlorine, and the demand
is 6 mg/l, then the alkalinity reduction is 1.3 parts
of alkalinity for each part of chlorine. This rule is
reliable.
|